You just got your new Bontrager Jackalope and Hodag combo set up and ready to go, now it’s time to hit the trails. But what about tire pressure? If you’re like most riders willing to brave the cold, you probably inflate your tires inside your garage, basement, shop, etc. After all, it can be much easier to work small parts like presta valves when you can actually feel your fingers.
Most people know that air pressure drops when it’s cold, but how much? If you’re checking your tire pressure with a gauge at 70ºF, your psi may drop by as much as 4 pounds by the time you get outside. While 4 psi might not sound like much, if you’re running 8 psi in a fat bike tire, losing half of your air pressure is a pretty big deal.
Check out Bontrager’s conversion chart next…
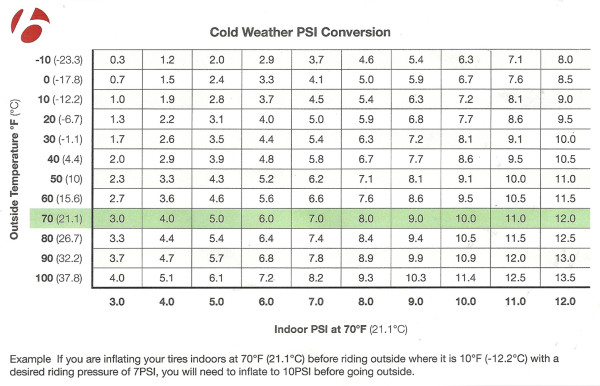 Starting with the Ideal Gas Law, Bontrager put together the Conversion chart above based on the air volume of a Hodag Tire (3.8″) on a Jackalope rim (80mm). Other 3.8″ fat bike tires mounted to 80mm rims should have similar numbers, but wider tires will require some additional math and the use of one of the many PV=nRT calculators found online. That PV=nRT is the Ideal Gas Law where P is pressure, V is volume of gas, n is the amount of gas in moles, R is the ideal gas constant, and T is the all important temperature in this case.
Starting with the Ideal Gas Law, Bontrager put together the Conversion chart above based on the air volume of a Hodag Tire (3.8″) on a Jackalope rim (80mm). Other 3.8″ fat bike tires mounted to 80mm rims should have similar numbers, but wider tires will require some additional math and the use of one of the many PV=nRT calculators found online. That PV=nRT is the Ideal Gas Law where P is pressure, V is volume of gas, n is the amount of gas in moles, R is the ideal gas constant, and T is the all important temperature in this case.
Using the chart above, it should be a bit easier to arrive to your destination with the desired pressure right off the bat. Obviously temperatures on the trail may change, and you need to know what the temperature is when you’re inflating your tires. This is definitely not for the riders who simply go by feel, but for those looking to run specific psi it could be pretty useful.
